Abstract
Background
Plasma cell disorders (PCD) are at risk of inadequate immune responses to COVID-19 vaccines due to recognised humoral and cellular immune dysfunction which is multi-factorial and related to host and disease factors. With an estimated risk of 33% mortality from contracting COVID-19 in this population, protection with an anti-SARS-CoV-2 vaccination is critical. Initial extension to vaccination intervals in the United Kingdom to 12 weeks in December 2020 led to concerns that PCD patients would be left vulnerable for an extended period.
Methods
A clinical audit was performed on measured serological responses in PCD patients after first and second doses of the BNT162b2 and ChAdOx-1 nCoV-19 vaccines. Antibody levels were measured using Elecsys Anti-SARS-CoV-2S assay (Roche) for quantitative detection of IgG Abs, specific for the SARS-CoV-2 spike-protein. Positive cut-off of 0.80 U/mL defined serological response. Testing was performed at (or closest to) 4 and 8-weeks post-dose. Baseline nucleocapsid Ab results were available from previous screening in a subset of patients. All patients on CIT underwent 4-weekly swabs. Clinical information was retrieved from medical records.
Results
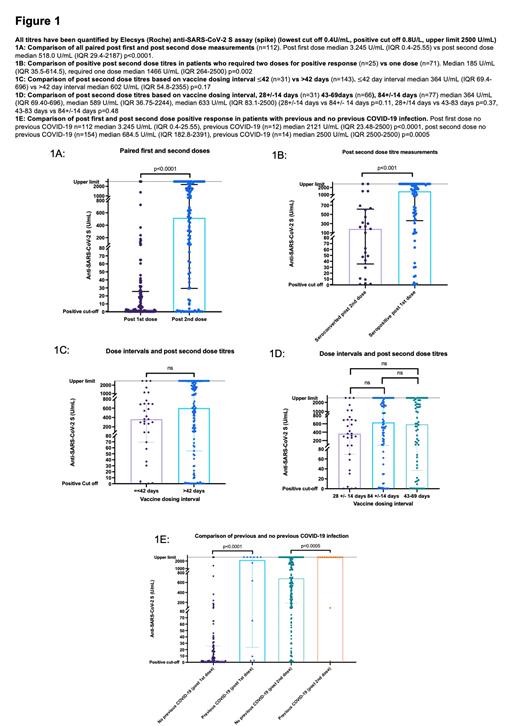
188 PCD patients (155 multiple myeloma, 18 amyloid, 10 SMM/MGUS, other 5 PCD), median age 64 (range 32-84), had serological assessment after both vaccine doses. Fourteen with previous COVID-19 infection were excluded. Of 174 patients, 112 were tested after first dose. 88% (153) were on chemo-immunotherapy treatment (CIT). Seropositive rate after first dose was 63% (71/112); of those with available negative baseline antibody test, 62% (31/50) seroconverted. After second dose, 89% (154/174) were seropositive; of those with negative baseline antibody, 90% (61/68) seroconverted. Expectedly, paired median titres after second dose were significantly higher than post first dose (n=112, 3.245 U/mL (IQR 0.4-25.55) vs 518 U/mL (IQR 29.40-2187) p<0.0001) (Figure 1A). Of 41 patients seronegative after first dose, 25 (61%) seroconverted after second, though with lower titres than those only requiring one dose (Figure 1B). Active CIT, disease response less than PR, >=4 lines therapy, light-chain disease, male gender and not responding to first dose were significant factors for not responding to two vaccine doses. We explored <400 U/mL as sub-optimal response (in keeping with upcoming booster study eligibility, OCTAVE-DUO(1), also encompassing the lower quartile of reported healthy controls(2)), which included 43% (75/174) patients. Age 70 years, male gender, >=4 lines of treatment were independent predictors of less-than-optimal response (anti-CD38 CIT of borderline significance). Importantly, vaccine dosing intervals classified as =<42 vs >42 days (Figure 1C) or 28 +/- 14 days vs 84 +/- 14 days (excluding n=66 in neither) (Figure 1D) did not show difference in both definitions of response, neither did vaccine type. Fourteen with previous COVID-19 infection responded to one vaccine dose, median titres 2121 U/mL (IQR 23.48-2500)) rising to median 2500 U/mL (IQR 2500-2500) after second dose (Figure 1E), significantly higher than those without previous infection.
Conclusion
Serological response to COVID-19 vaccine is lower in PCD patients than reported healthy controls at 63% after first dose, rising to 89% after second dose, despite extended dosing intervals. PCD patients should be prioritised for shorter intervals, as we show that patients seronegative after first dose, respond after second dose. Further work in PCD is needed to understand how Ab levels correlate to neutralisation capability, cellular responses, protection from infection and how long seroconversion lasts to better define correlates of protection. A booster vaccination or prophylactic passive antibody strategy may be required for those identified at risk, shown not to have responded to two vaccine doses or to have less-than-optimal response. Results from these trials will be eagerly awaited.
References:
1. University of Birmingham. About the OCTAVE Trial 2021 [Available from: https://www.birmingham.ac.uk/research/crctu/trials/octave/patients-and-public/about-octave.aspx. Accessed 1 st August 2021.
2. Avivi I, Balaban R, Shragai T, Sheffer G, Morales M, Aharon A, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol. 2021.
Wechalekar: Amgen: Research Funding; Alexion, AstraZeneca Rare Disease: Consultancy; Caelum Biosciences: Other: Clinical Trial Funding; Janssen: Consultancy; Takeda: Honoraria; Celgene: Honoraria. Popat: AbbVie, BMS, Janssen, Oncopeptides, and Amgen: Honoraria; Takeda: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Abbvie, Takeda, Janssen, and Celgene: Consultancy; Janssen and BMS: Other: travel expenses. Rabin: BMS / Celgene: Consultancy, Honoraria, Other: Travel support for meetings; Takeda: Consultancy, Honoraria, Other: Travel support for meetings; Janssen: Consultancy, Honoraria, Other: Travel support for meetings. Yong: BMS: Research Funding; Amgen: Honoraria; GSK: Honoraria; Takeda: Honoraria; Janssen: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Autolus: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal